Understanding the molecular processes that control both collective and individual cell invasion in head and neck cancer
Why investigate invasion in H&N cancer?
The consequences of highly invasive HPV-negative head and neck squamous cell carcinoma (HNSCC) in the clinic include positive surgical resection margins, which are directly linked to locoregional relapse and metastatic expansion. However, tumor infiltration presents as a variety of invasive forms in HNSCC, including collective or single cell invasion, revealing a complex wiring of invasive phenotypes. Importantly, the pattern of invasion is identified as an independent prognosticator on the tumor invasive front. By decoupling collective and single cell invasive programs, novel therapeutic options targeting host deadly tumor cells on the invasive front may deliver significant survival benefits.
How can we study invasion?
Patient-derived organoid (PDO) models offer a unique opportunity to investigate therapeutically relevant phenomena as tumors grow. By embedding PDOs in extracellular matrix networks that mirror features of the local underlying tissue structure and function identified in patients, molecular profiles regulating invasion can be uncovered. To do this, we first created an in vitro panel of invasive HPV-negative HNSCC PDOs that mimicked the invasive patterns seen in original tissues. We next used high-throughput single-cell mRNA transcriptomics to investigate the differences in expression profiles between invasive and non-invasive circumstances, as well as in a collective versus single cell invading PDO model.
YAP-centered program directs collective invasion.
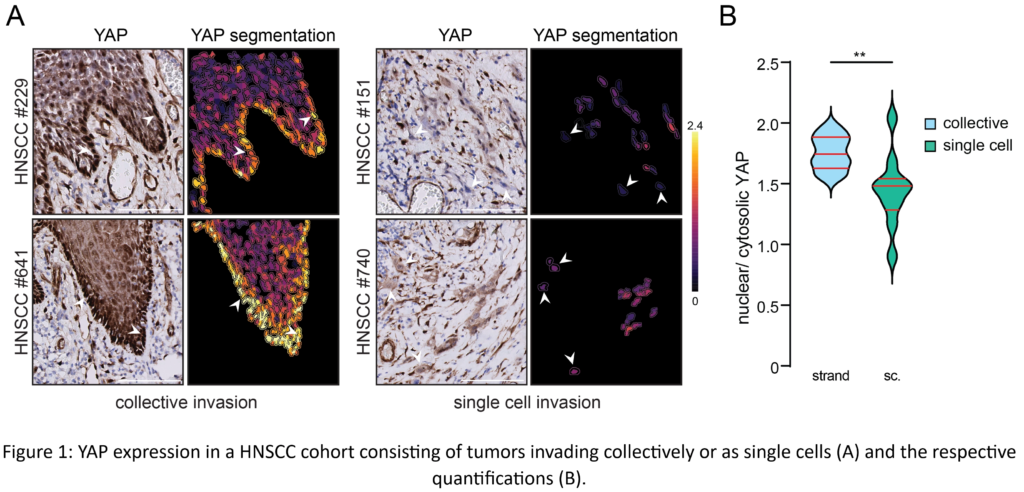
Our findings show that invasive HNSCC PDOs upregulate a YAP-centered transcriptional pathway regardless of invasion modality. However, we discovered that collectively invading PDO cells have larger quantities of YAP transcription targets than single-cell invasion. These invading strands are distinguished by enhanced nuclear YAP and collagen fiber alignment at the invasive front (Figure 1A and B). Interestingly, our findings indicate that the activation of extracellular matrix receptors on tumor cells dominates the regulation of the Hippo pathway during collective invasion, even in the presence of established cell-cell junctions.
Invasion routes and clinical implications
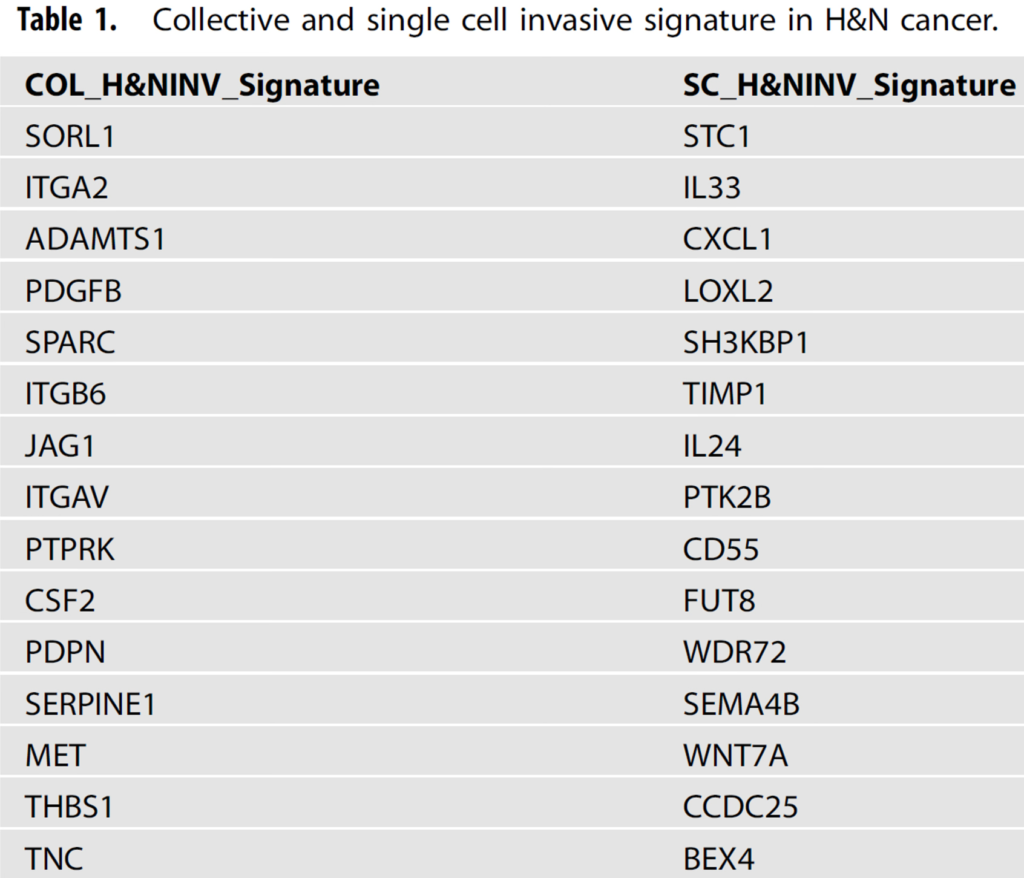
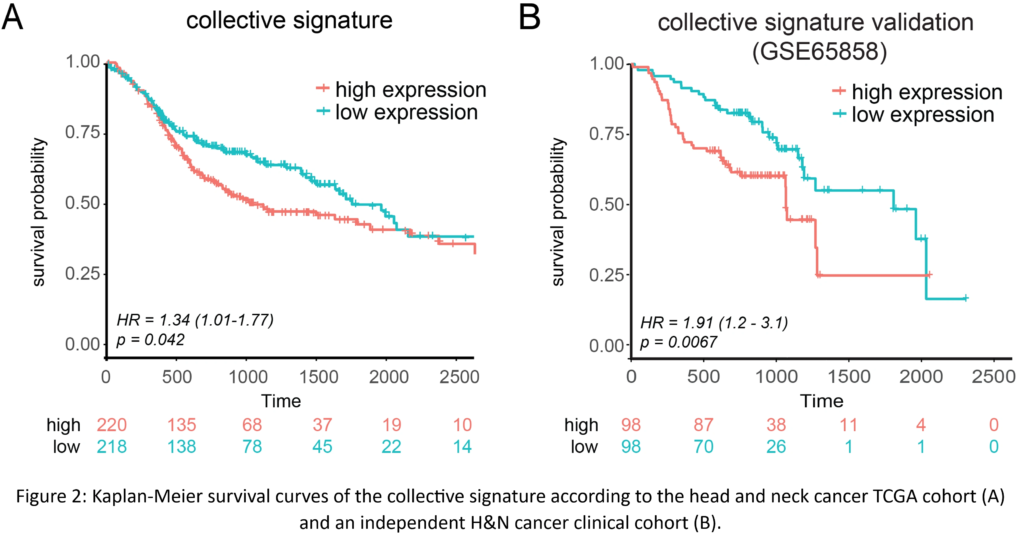
Our research revealed that collective invasion is defined by a combination of adhesion and migratory YAP-centered pathways. We were able to produce distinct invasion signatures, with the collective signature demonstrating predictive usefulness in HNSCC clinical cohorts (Table 1 and Figure 2A and B). Furthermore, the collective signature acted as a prognosticator in seven different solid cancer types, revealing new signs of similar invasive processes.
Conclusions
Finally, we created HNSCC PDOs with varied invasion patterns in 3D collagen matrices. Individual cell invasion is distinguished by immune-like programs, but collective invasion is fueled by a YAP-centered adhesion and migration program. Finally, the collective signature has predictive value in a variety of solid cancer types, including HPV-negative HNSCC. This finding opens up new therapy options and improves our understanding of HNSCC invasion dynamics.
